Oils and extracts to powder
Schematic flow
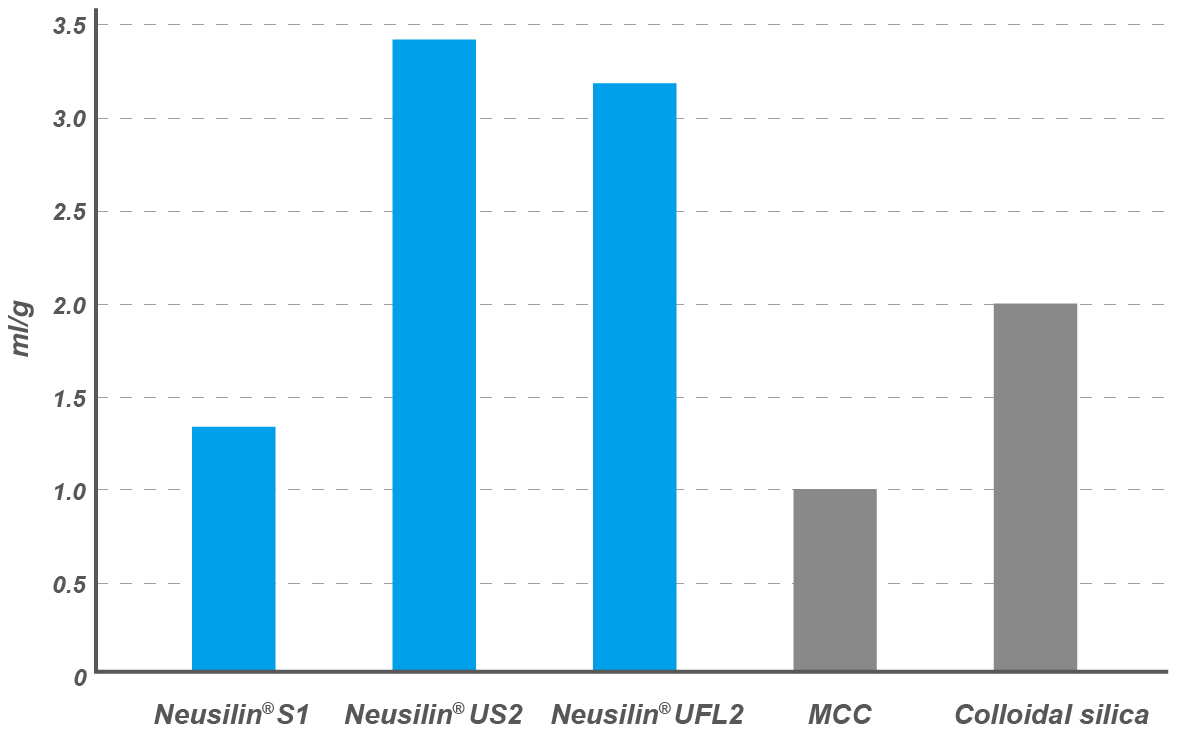
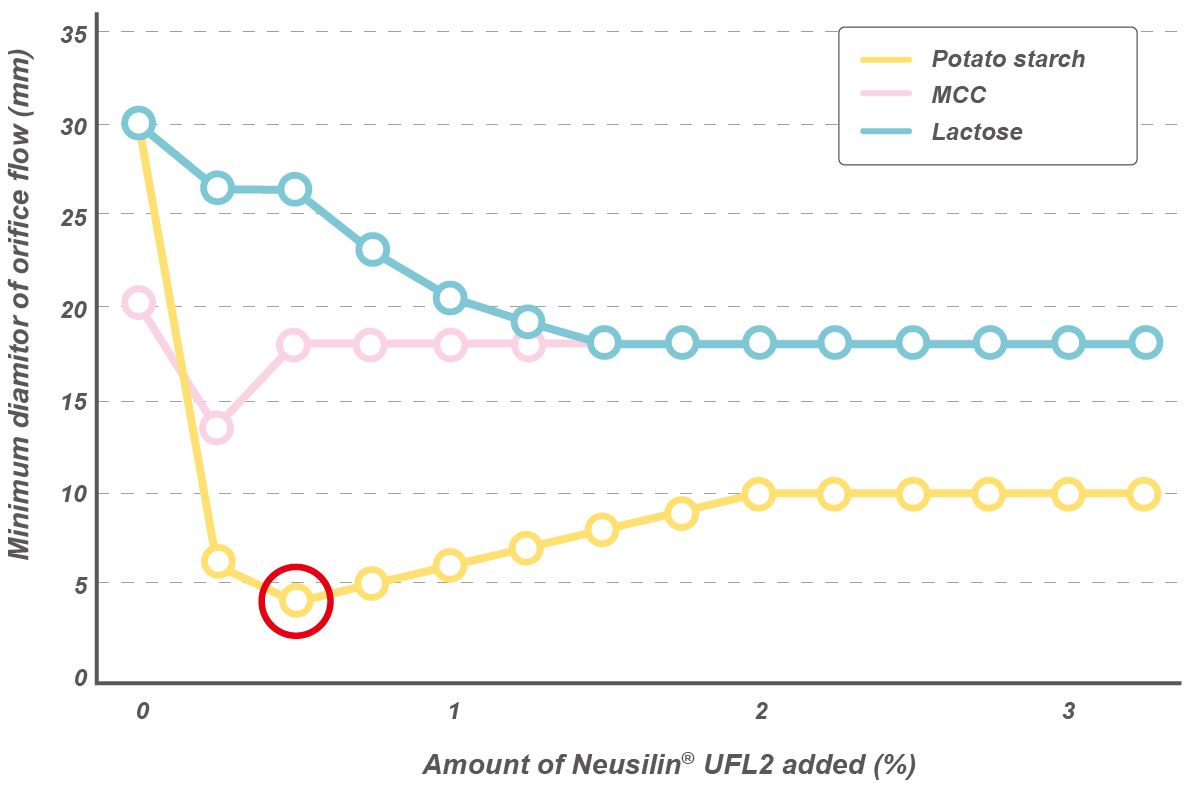
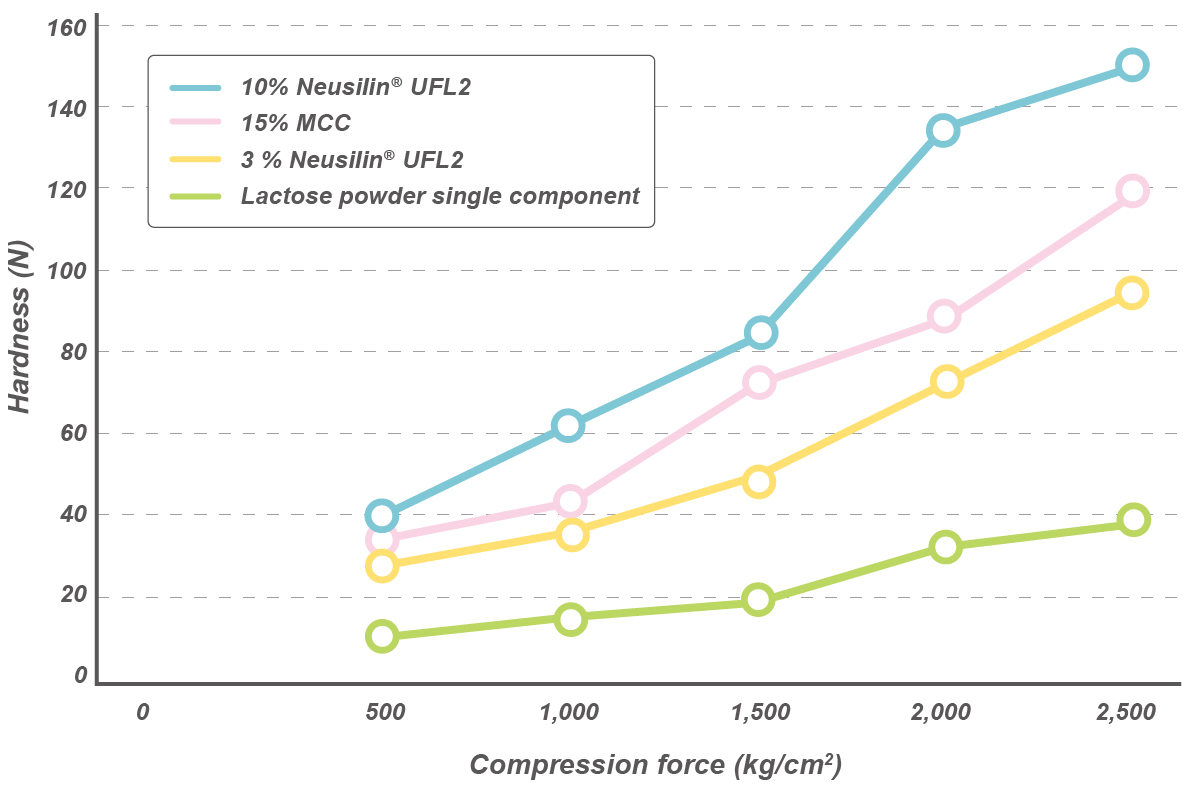
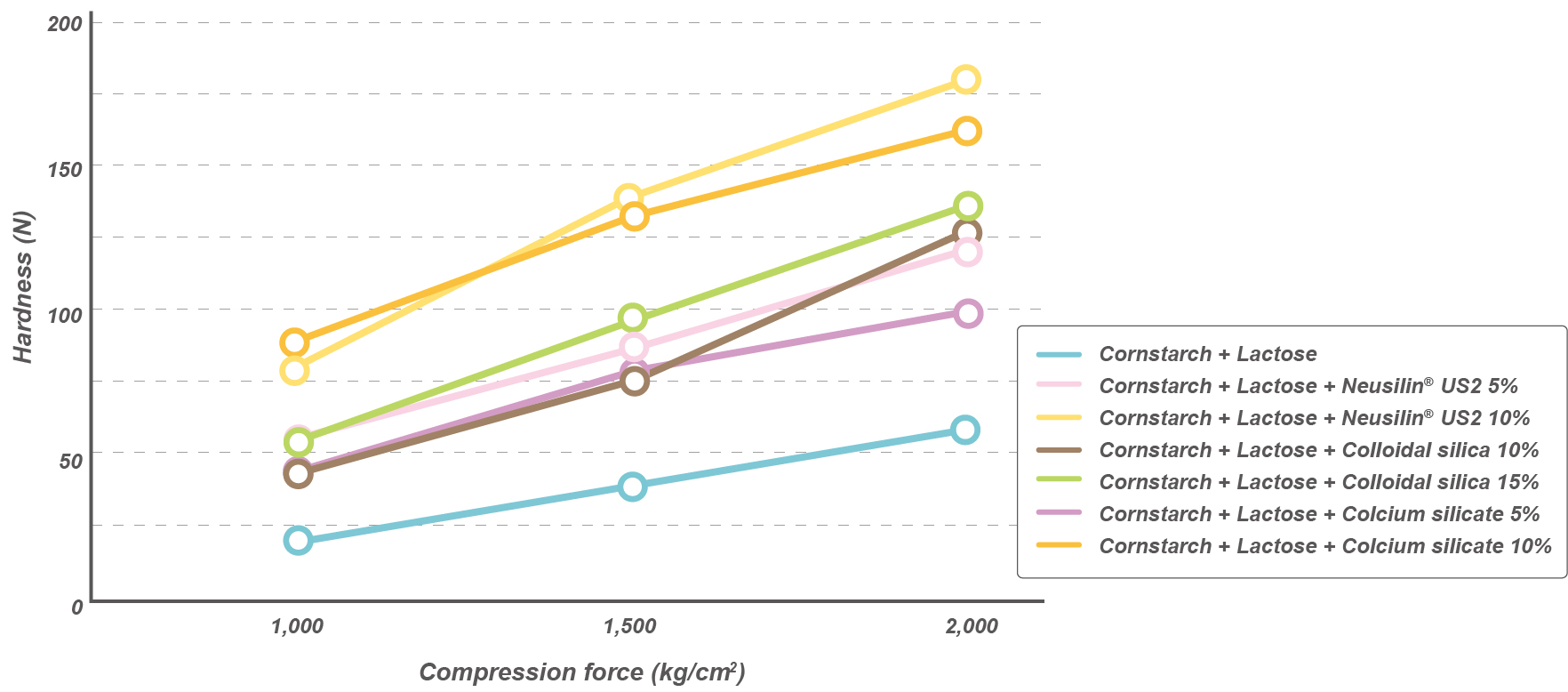
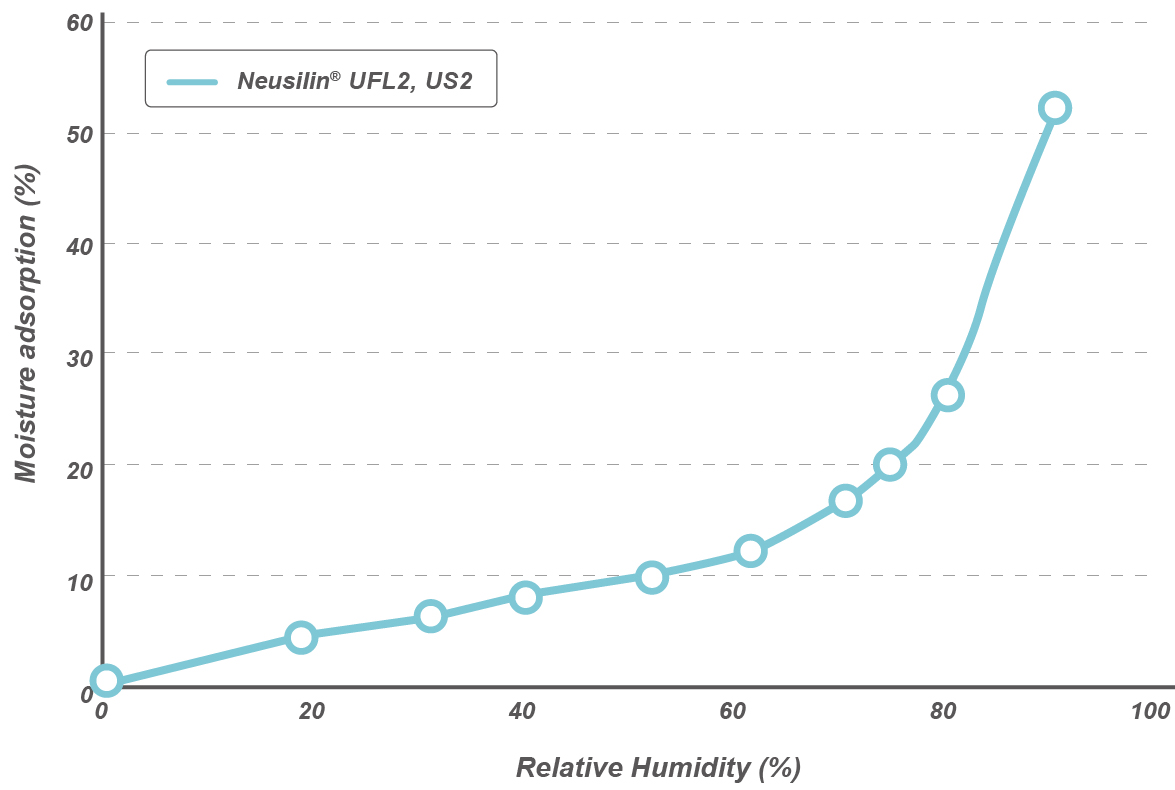
Oil adsorption capacity
Neusilin® US2 and UFL2 grades show higher oil adsorption capacity* when compared to MCC or colloidal silica.
*Linseed oil direct adsorption
Free flowing powder of linseed oil
Neusilin® US2 +30% linseed oil, Dry at 50ºC
Linseed oil tablet, Ø11.3mm, 125N at 500 kg/cm2
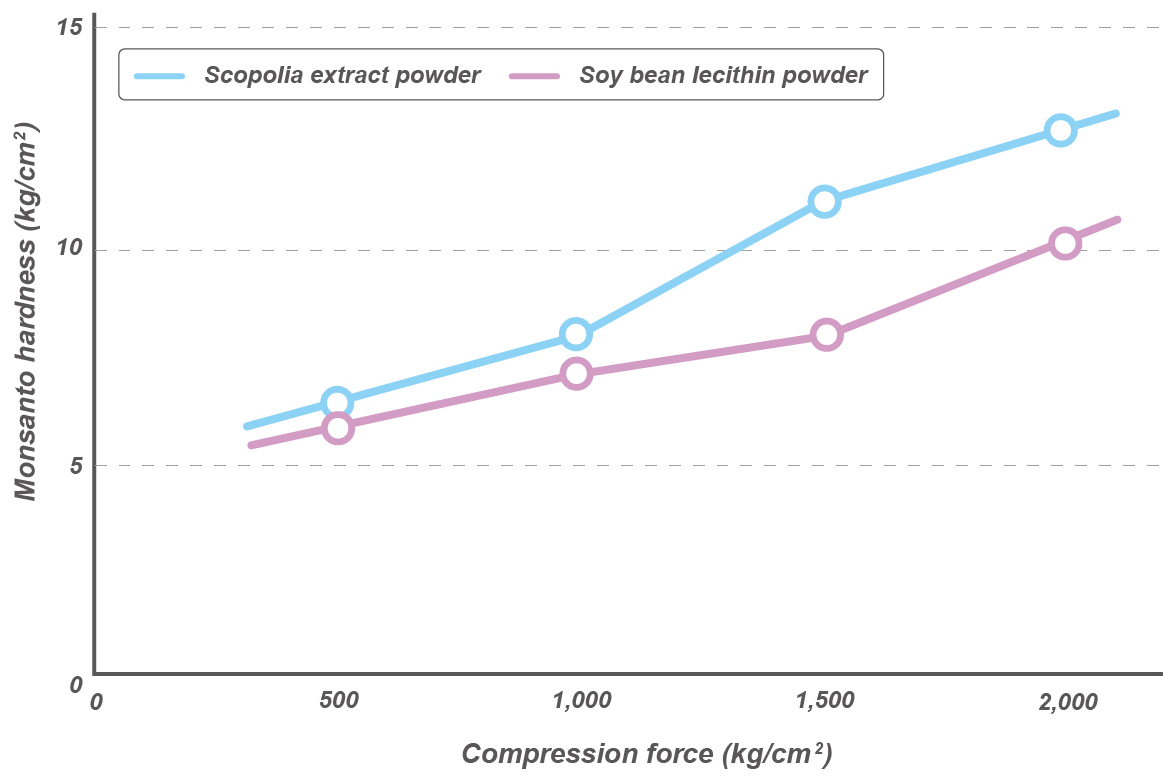
Tablets of Scopolia extract and soybean oil UFL2
A mixture containing 25% Scopolia extract or soybean oil and 25% UFL2 was compounded with equal amount of lactose.
This mixture was subjected to static compression and tabletting. We found no adhesion to pestle and mortar and the compressibility was good. The tablet did not exude the extract or oil during storage.
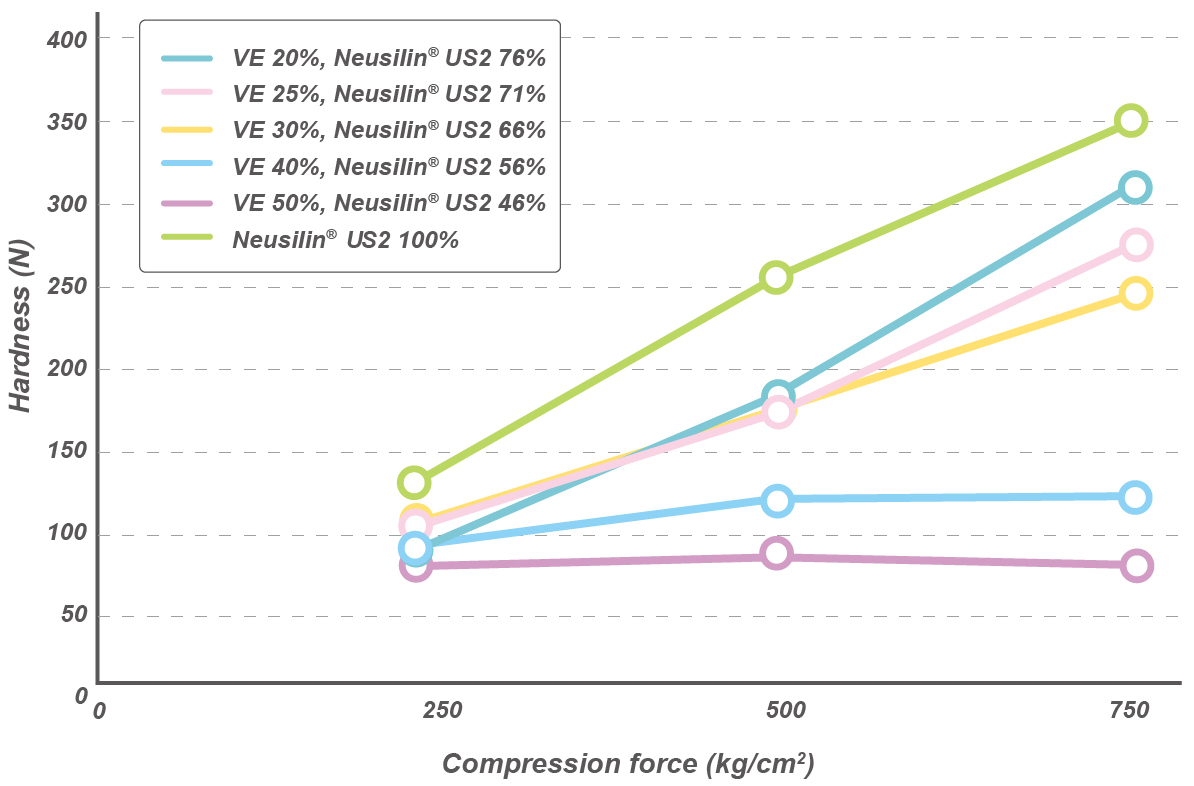
Tablets of Vitamin E US2
An ethanol solution of tocopherol acetate (VE) 20-50% was compounded with proportional amount of Neusilin® and mixed well.
To this mixture, 3% croscarmellose sodium and 1% magnesium stearate were added before tabletting.
High quality tablets with a load of up to 30% vitamin E can be prepared with Neusilin® US2.
Solid dispersion
Formulating poorly water soluble drugs by solid dispersion leads to a remarkable improvement in dissolution and bioavailability. Neusilin® can potentially resolve problems associated with tabletting and improve efficiency of solid dispersion.
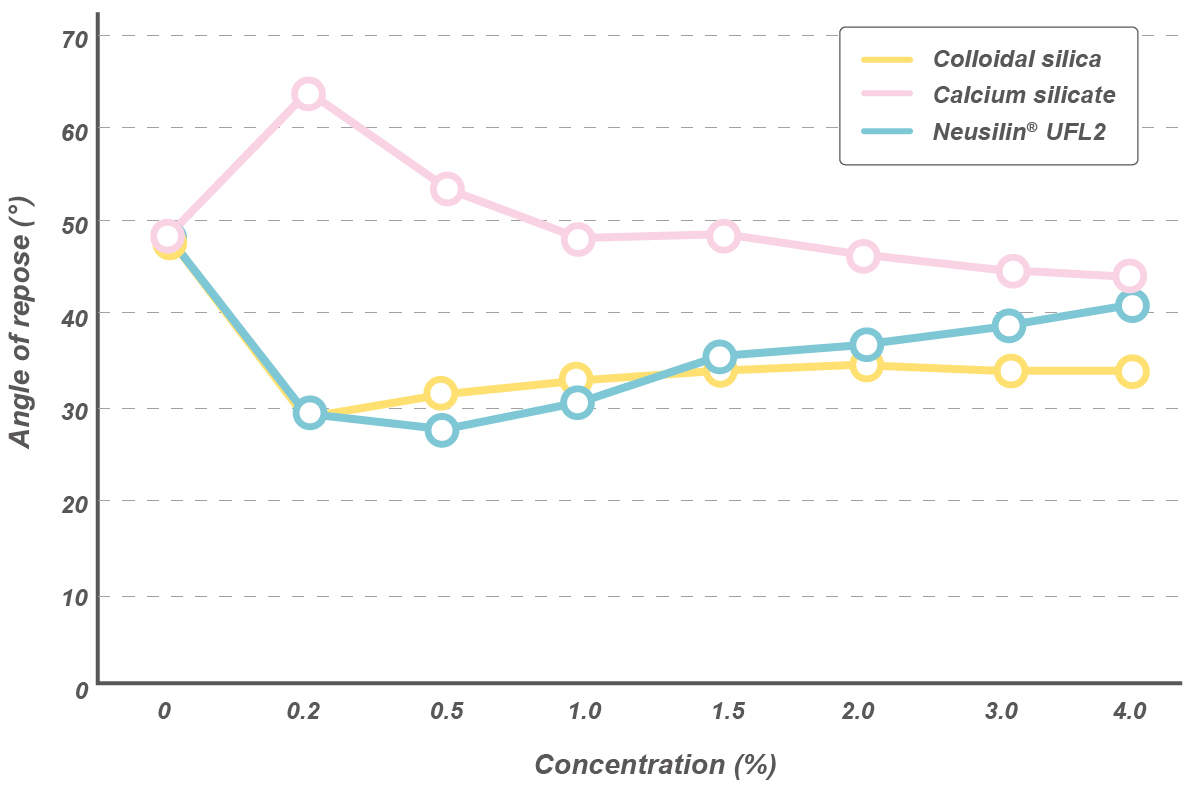
Key advantages of Neusilin® as an adsorbent
- Flowability improvement
- High quality tablets at low compression forces
- High specific surface area
- High adsorption capacity
- Higher API load
- Restriction on reversion of amorphous form to crystalline state
- Inert core material
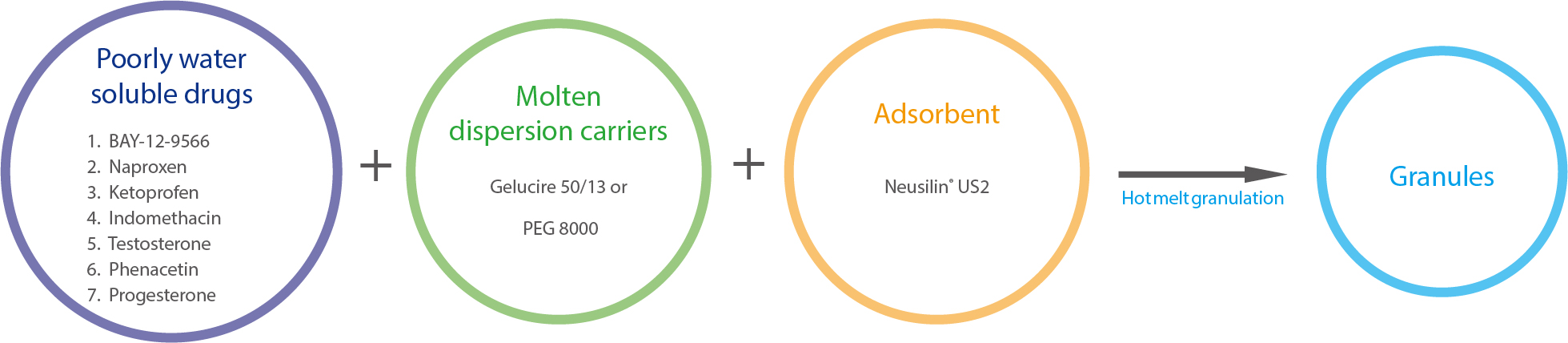
Case Study I US2
Solid dispersion granules
Gupta MK, et al. Hydrogen bonding with adsorbent during storage governs drug dissolution from solid-dispersion granules. Pharm Res. 2002; 19:1663-72.
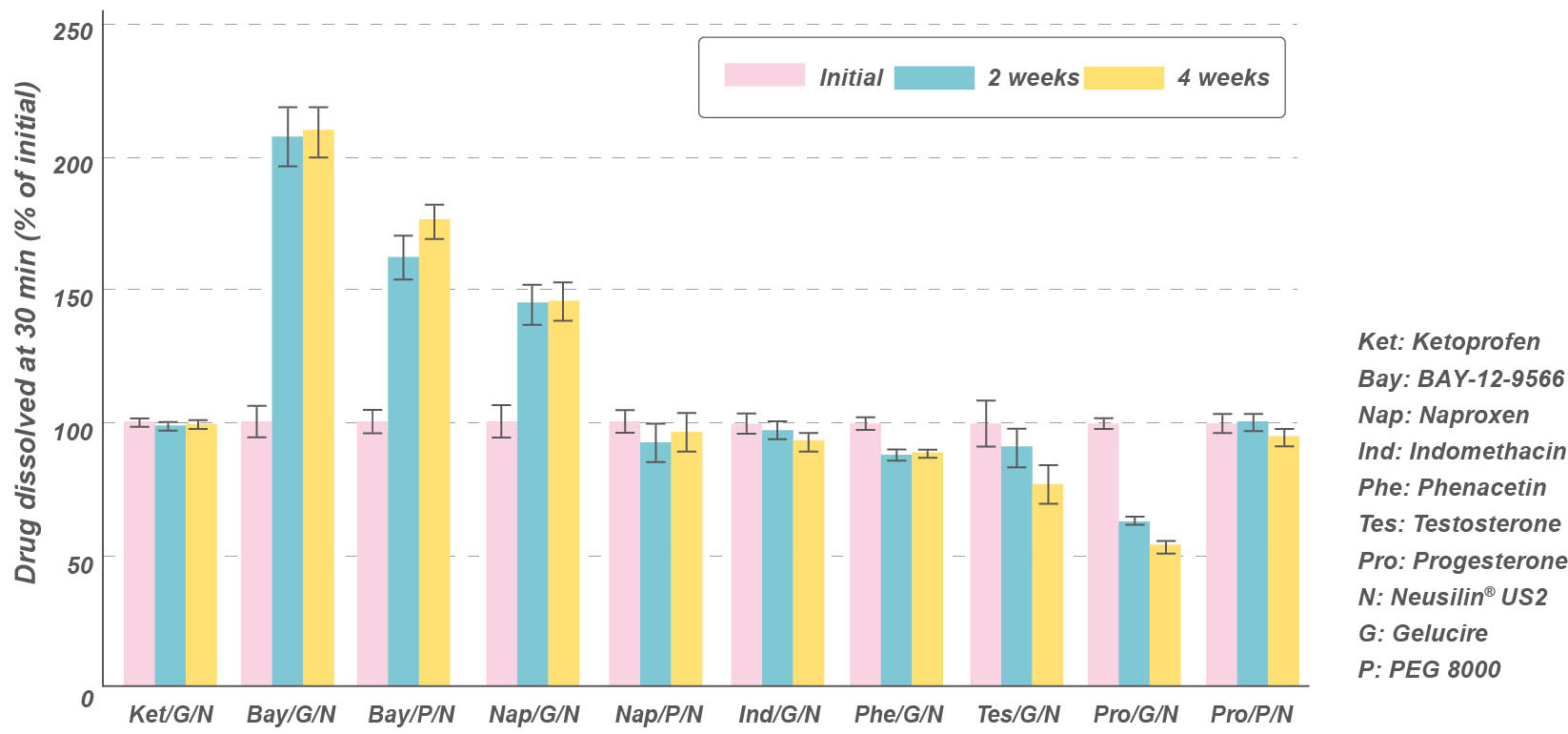
Dissolution profile of solid dispersion granules
Comparison of drug dissolution (after 30 min) from initial and stored solid-dispersion granules using USP Type II apparatus at 50 rpm.
Data are shown for drug dissolution (% of initial) from solid-dispersion granules after storage at 40ºC/75% RH (Gupta et al, 2002)
Case Study II US2
Solid SEDDS (self-emulsifying drug delivery systems) formulation
Use of Neusilin® as adsorbent carrier to convert liquid SEDDS to solid SEDDS
1. Glyburide SEDDS tablets
Mura P, et al. New solid self-microemulsifying systems to enhance dissolution rate of poorly water soluble drugs. Pharm Dev Technol. 2012; 17:277-84
Self micro emulsifying formulation was prepared by adding under continuous stirring Tween 20 and Labrafac Hydro® WL (oil phase) and then distilled water to glyburide solubilized in Transcutol®. Glyburide tablets were prepared by direct compression. The preparation with Neusilin® US2 resulted in improved flow, compact tablets and improved dissolution profile.
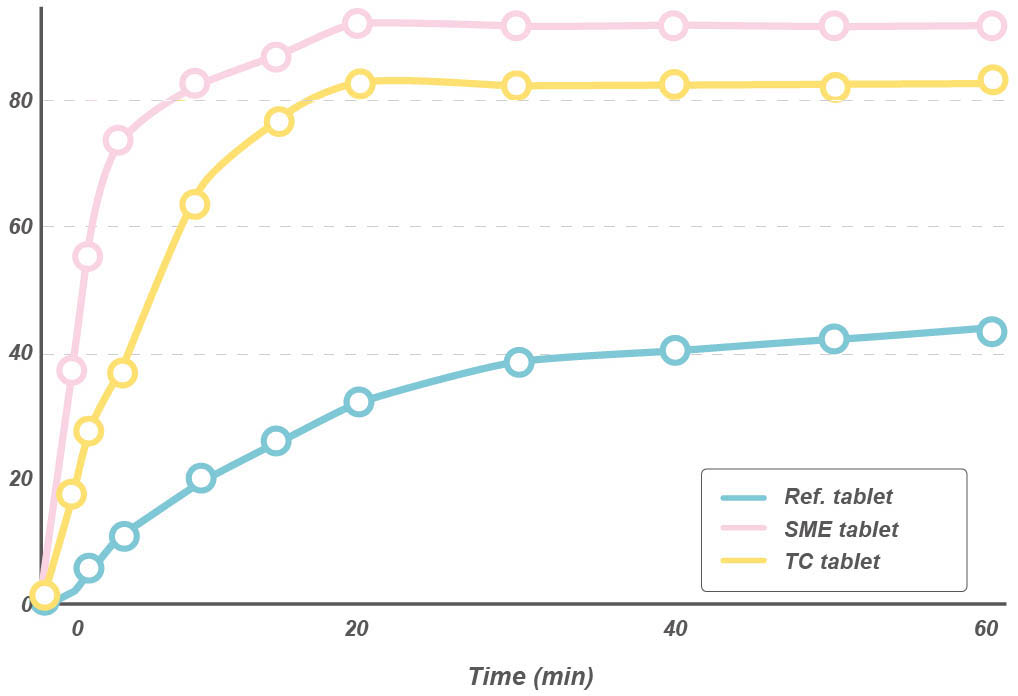
Dissolution profile of glyburide preparation
Glyburide (GLY) dissolution profile from the different tablet formulations (Ref. Tablet – commercial GLY formulation; SME tablet – glyburide SME formulation consisting of Labrafac Hydro® as oil phase, Tween 20 as surfactant and Transcutol® as co-surfactant; TC tablet – glyburide formulation consisting of Transcutol® (TC) glyburide.
2. Solid SEDDS of paliperidone
Kanuganti S, et al. Paliperidone-Loaded Self-Emulsifying Drug Delivery Systems (SEDDS) for Improved Oral Delivery. J Disp Scie and Technol, 33:506–515, 2012
Optimized SEDDS formulation containing oleic acid, Tween 80 and Capmul® MCM L8 was adsorbed onto Neusilin® US2 to produce solid SEDDS (SEDDS-N). To understand the release behavior of paliperidone from solid SEDDS and pure drug, in-vitro dissolution test was performed.
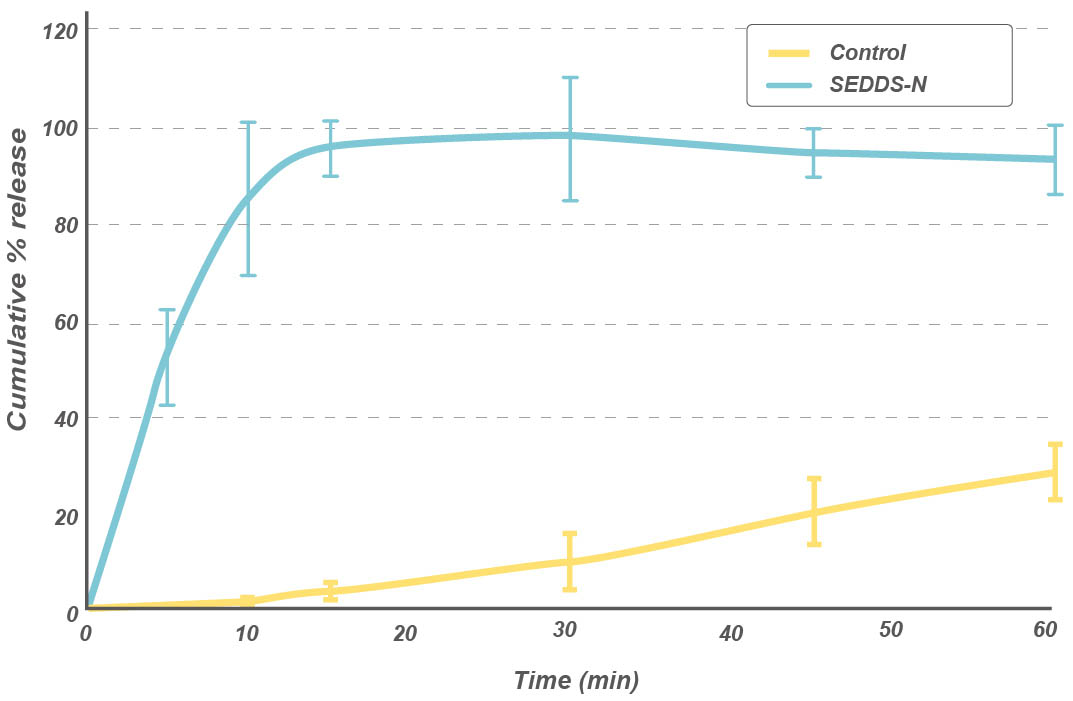
Dissolution profile of paliperidone powder
The drug release was faster and the dissolution efficiency was higher for the solid SEDDS compared to that of crystalline form.
Case Study III US2
Hot melt extrusion (HME)
Maclean et al. Manufacturing and performance evaluation of a stable amorphous complex of an acidic drug molecule and Neusilin®. J Pharm Sci. 2011; 100:3332-44.
HME of Sulindac-Neusilin® Drug Complex
Blends of Sulindac-Neusilin® in 1:1 and 1:2 (w/w ratio) were prepared by HME at 200ºC.
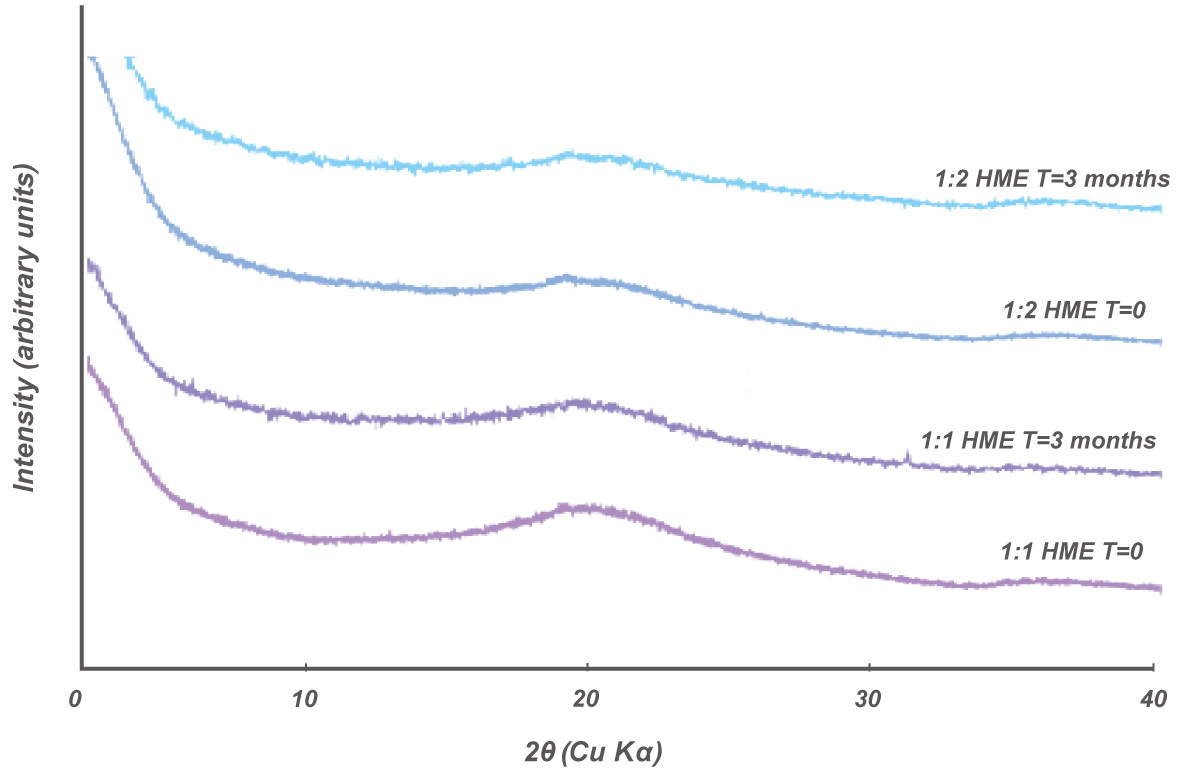
Physical / Chemical Stability of Sulindac-Neusilin® HME Complex
The HME samples remained amorphous after 3 months of storage at 40ºC/75% RH. The samples were found to remain amorphous for more than one year at ambient conditions.
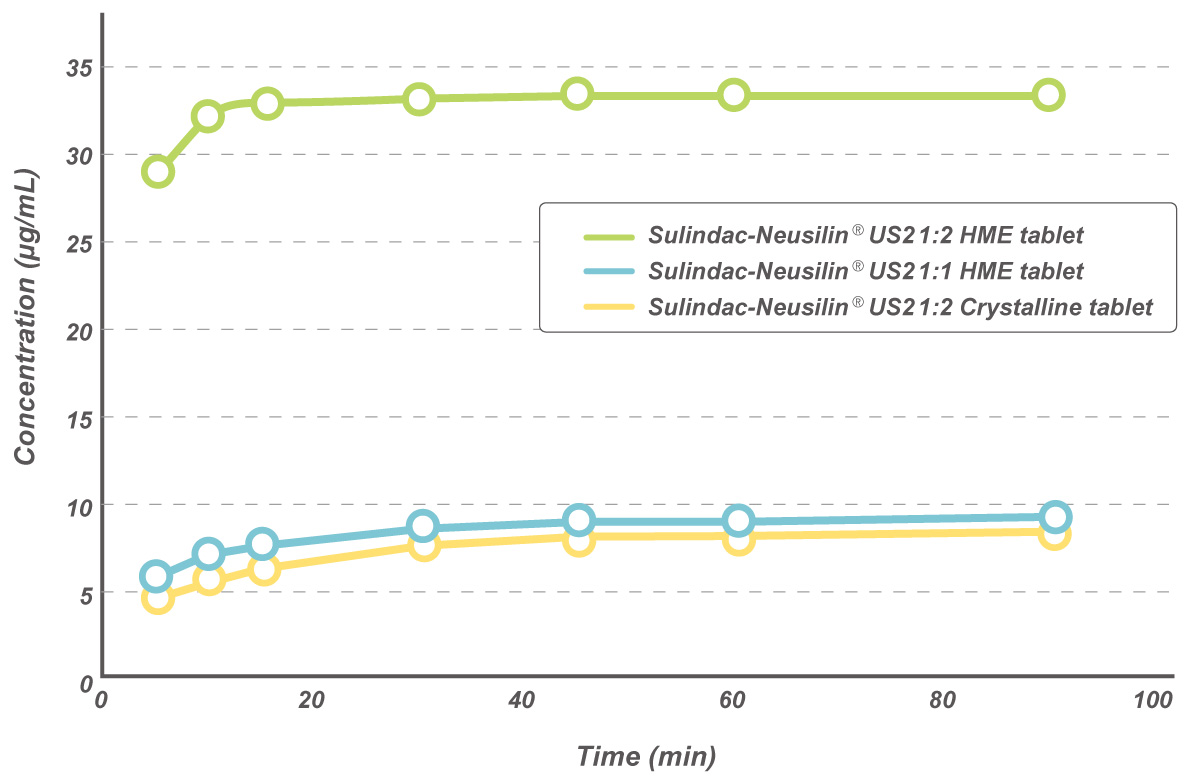
Sulindac-Neusilin® HME tablets
Sulindac-Neusilin® 1:2 HME tablets showed 100% release in 90 minutes as against 9% release of Sulindac-Neusilin® crystalline tablets.
Dissolution profiles of HME Sulindac-Neusilin® tablets